ACTION AREA 1: HPV Prevention Via Gender Neutral Vaccination Programmes
HPV-related cancers and genital warts can be prevented by HPV vaccination. This is most effective when administered in adolescence, before exposure to the virus through sexual activity. There is, however, value in vaccinating older teenagers and young adults, at least up to the age of 26 because it can protect against a new infection or re-infection and block transmission to a new partner.25 In the USA, HPV vaccination is now recommended for all men and women up to the age of 26. There is also some evidence supporting the vaccination of all women up the age of 30 (or older) at the same time as a cervical screen;26 this approach is currently being considered in Sweden.27
When HPV vaccination programmes were first introduced, three separate doses were recommended to ensure optimal immunity. It has since been recognised that two doses are sufficient and research is currently underway in Costa Rica and elsewhere to determine the efficacy of a single dose regime. This follows evidence that women in current programmes who did not receive the recommended number of vaccinations nevertheless appear to be well-protected.28 If a single dose proves to be effective, this would have a major impact worldwide, making it easier and cheaper to deliver vaccination programmes. The public health benefits in low-income countries would be particularly significant.
The impact of HPV vaccination on cancer incidence is clear and significant. A large-scale study in Scotland found that, compared with unvaccinated women born in 1988, vaccinated women born in 1995 and 1996 had an almost 90% reduction in the highest-risk cervical pre-cancers (Cervical Intraepithelial Neoplasia [CIN], i.e. cervical pre-cancer, grade 3 or worse), an almost equivalent reduction in CIN grade 2 or worse and a near-80% reduction in CIN grade 1.29
100% vaccine effectiveness was demonstrated over 12 years in four Nordic countries: no cases of high-grade cervical dysplasia linked to HPV types 16 or 18 were found in a large sample of vaccinated women.30 The incidence of genital warts has also been significantly reduced by HPV vaccination.31 The US Food and Drug Administration (FDA) has recently approved vaccination as a means of preventing head and neck cancers caused by HPV.32
The almost unique potential of HPV vaccination for improving public health is clear. Compared to many other cancer prevention interventions – such as tobacco control, reducing risky alcohol consumption, increasing physical activity or tackling obesity – it is easy-to-deliver, has an immediate impact and is highly effective.
The Case for Universal HPV Vaccination
The vaccination of females alone will not provide effective protection for men against HPV infection. Unvaccinated females – such as those too old to have been offered routine vaccination or women who, although eligible, did not receive it – remain at risk of infection and can pass the virus on. In Europe as a whole, only about 4% of all women are estimated to have been vaccinated; in Northern Europe, the best-performing region, the figure rises to only 8%.33 Although these statistics are expected to improve over time as more women receive the vaccine, they do indicate the extent of the HPV infection ‘reservoir’.
Heterosexual men living in countries with relatively high female HPV vaccination rates remain at risk of infection from unvaccinated women locally, as well as from women from countries with low-uptake, or no, vaccination programmes. Men who have sex with men are at particular risk as they are completely unprotected by female-only HPV vaccination programmes, even in countries with very high levels of uptake by girls. It should be noted that HPV can also be transmitted between unvaccinated female sex partners.34
The case for vaccinating boys against HPV is reinforced by the fact that men have a poorer immune response to HPV infection than women. Men are less likely to seroconvert following infection, leaving them more vulnerable to re-infection.35 HPV infection rates appear to stay constant in men, independent of age,36 whereas HPV prevalence in women is highest during 18–24 years of age and then decreases until middle age.
Vaccinating both sexes against HPV (known as ‘universal’ or ‘gender-neutral’ vaccination) provides much greater levels of protection for everyone. Its efficacy lies in preventing the transmission of HPV between the sexes and in same-sex couples, reducing the circulation of the virus overall and creating what is termed ‘herd protection’. Universal HPV vaccination is an especially important public health strategy in countries where vaccination uptake in girls is relatively low.
Universal HPV vaccination is consistent with the fundamental human right to the highest attainable standard of health. Excluding men is unfair, and in some jurisdictions possibly unlawful on grounds of sex discrimination, as it makes a potentially life-saving intervention unavailable solely on the grounds of sex. Universal vaccination would also lead to greater equity between the sexes, between countries, and between income groups (in the absence of national programmes, wealthier families are choosing to purchase vaccines for their sons or daughters).
Universal HPV vaccination programmes also remove from females the sole responsibility for preventing HPV infection and help to overcome stigma about female vaccination based on unfounded concerns that it might encourage ‘promiscuity’. Universal programmes are also more resilient to unexpected falls in uptake, for example as a result of unfounded scares spread by ‘fake news’.37
Vaccinating both sexes against HPV provides an effective and faster approach to preventing or reducing the incidence of cancers and other HPV-related diseases. A universal approach could make the elimination of HPV-caused diseases possible even with moderate levels of vaccination uptake (50-75%).38
The European Centre for Disease Prevention and Control (ECDC) has suggested that if the objective of HPV vaccination is to prevent all HPV-caused disease, rather than cervical cancer alone, then universal vaccination may be a cost-effective option.39 In 2018, the highly-influential Joint Committee on Vaccination and Immunisation (JCVI), the UK government’s vaccination advisory committee, concluded that vaccinating both boys and girls is cost-effective, even when over 80% of girls are vaccinated, if the impact of HPV-related diseases in the long-term is taken into account.40 It should be noted, however, that cost-effectiveness modelling is contingent on highly variable assumptions (e.g. vaccine price, the proportion of cancer cases attributable to HPV and the costs of treatments) and, in any event, should not be the sole factor in decision-making about access to vaccines. Issues of equity, ethics and patient experience must also be taken into account.41
25 EU countries now provide national HPV vaccination programmes for girls. (The exceptions are Romania and Poland, although Poland plans to introduce a girls’ programme in 2021.) 40 out of 54 countries across the WHO European region as a wholea have national HPV vaccination programmes for girls.42 Boys are currently included in national HPV vaccination programmes in ten out of 27 EU countries and in eight other countries in the rest of the WHO European region. Finland, France, Hungary, the Netherlands, Poland, Portugal, Slovenia and Sweden have made a commitment to introduce HPV vaccination for boys. A total of 26 countries are therefore currently, or will be, including boys in their national HPV vaccination programmes; this represents almost half (48%) of all countries in the region.
Figure 1. Countries in WHO European region with national HPV vaccination programmesb
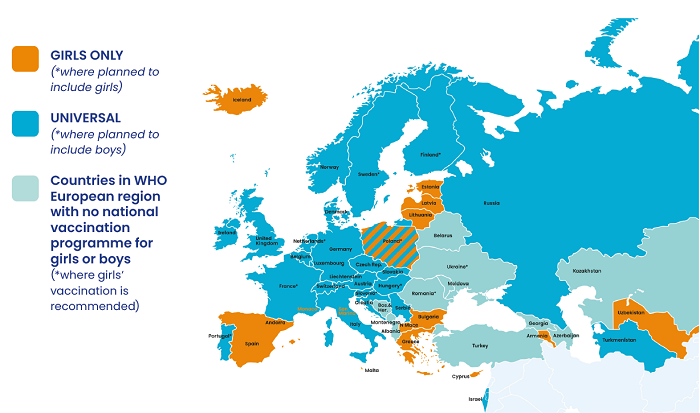
a Includes Liechtenstein, which is not a WHO Europe member.
b Information about vaccination policies in specific countries may not be complete as up-to-date information for every state in the WHO European Region is not readily available. The same caveat applies to the section below on cancer screening.
Generally, countries in Northern, Western and some parts of Southern Europe are more likely to have an HPV vaccination programme, as compared to Eastern European countries. With some exceptions, the HPV vaccination programmes that include boys are located in Northern and Western Europe.
Universal HPV vaccination programmes are also becoming more common outside Europe. It is thought that 42 countries worldwide are currently vaccinating both boys and girls against HPV, including Argentina, Australia, Barbados, Bermuda, Brazil, Canada, Guyana, New Zealand, Trinidad and Tobago and the United States.
A few countries, including France and the UK, have introduced HPV vaccination programmes targeted specifically at men who have sex with men and other high-risk groups, such as sex workers. Such programmes have relatively low levels of uptake and their users are generally of an age where they are highly likely to have already been exposed to HPV infection.43,44 While they cannot be seen as a substitute for universal vaccination, these programmes can play an important role if properly resourced, especially since it will take many years before HPV vaccination programmes currently delivered to adolescents protect adults at higher risk. Migrants are another high-risk group which could benefit from targeted vaccination (as well as cervical cancer screening) programmes.45
There is currently a short-term global shortage of HPV vaccine and the WHO has recommended that countries ‘should temporarily pause implementation of boy, older age group (>15 years) and multi-age cohort (MAC) HPV vaccination strategies until vaccine supply allows equitable access to HPV vaccine by all countries’.46 This is intended to support cervical cancer prevention programmes in mainly low-income countries. Fewer than one in three girls lives in a country in which HPV vaccine is in the national immunization schedule, and those at greatest risk for cervical cancer are least likely to have access, as only 13 low-income countries have so far introduced the vaccine.
The HPV vaccine shortage is expected to be resolved by 2023 (perhaps earlier if new vaccines manufactured in China become available), meaning that European countries have an opportunity to develop their plans over the next 2-3 years and have them ready for implementation in 2023 or soon after. In the meantime, European countries, and the EU itself, could offer support to low-income countries developing HPV prevention programmes.
Vaccination Uptake
HPV vaccination uptake in females varies significantly across EU countries – few meet the widely-accepted target of at least 80% coverage.47 In some countries, such as Bulgaria, France and Greece, vaccination rates are particularly low.
In Western and Southern Europe, about one-third of females in the targeted populations is estimated to have completed the full course of HPV vaccination. In Eastern Europe, the proportion is only one-fifth. However, in Northern Europe, the best-performing region, about two-thirds of eligible females have been vaccinated.
HPV vaccination rates can also vary widely within countries themselves. The UK, for example, has a high overall vaccination rate (just over 80%) but, at the local level, uptake varies between 50% and 95%.48 There is evidence of lower rates of uptake among ethnic minority communities and disadvantaged socio-economic groups in both Europe and the USA.49
Low vaccination uptake has a range of causes, including cost, restricted access to health services, concerns about vaccine safety and lack of service co-ordination, which need to be better understood for each country. But action is needed to improve HPV vaccine delivery systems (school-based systems generally have higher levels of uptake and are usually considered to represent best practice but other models, such as Portugal’s use of community health clinics, can also be very effective) and to reassure the public, using robust scientific evidence about vaccine safety. Guidance about best practice would be very helpful, especially for those countries wishing to launch, develop or expand their vaccination programmes.
The COVID-19 pandemic has significantly disrupted HPV vaccination programmes across Europe and will undoubtedly cause a dip in uptake, at least in the short-term. The European Federation for Colposcopy (EFC) and the European Society for Gynaecological Oncology (ESGO) suggest that HPV vaccination can continue in countries with no cases or sporadic cases of COVID-19 but should be delayed in countries with clusters of cases and/or community transmission and where the mobility of vaccine recipients and healthcare staff must be restricted to restrict transmission of the SARS-CoV-2 virus.50
The JCVI has advised that, for the UK, the priority is for all eligible children to receive at least the first dose of the HPV vaccine.51 The committee considers that the interval between the first and second dose can be extended by a number of years without compromising protection or the boosting effect of the second dose.
EFC and ESGO consider that careful planning is needed to handle the backlogs accumulated due to COVID-19 to minimise drops in mid- and long-term HPV vaccination coverage. Measures should be taken to complete vaccination schedules for those who have already started HPV vaccination, assuring an interval less than 12-15 months from the first dose. Some local vaccination teams have developed innovative ‘drive through’ HPV vaccination schemes to reach children who would normally have been vaccinated at school.52 There may also be a potential role during the pandemic for community pharmacists.53
CASE-STUDY: Drive-Through HPV Vaccination Clinics
COVID-19 meant that the school nursing service in the Isle of Wight in Southern England was unable to complete scheduled range of childhood immunisations in schools prior to their enforced closure. Recognising the vital need to vaccinate as many children as possible, the service worked with partner organisations to reconfigure the delivery system. Drive-Through immunisation clinics were quickly developed and launched.
‘Pods’ with power and hot water were established in local council car parks. Parents were contacted, the process was explained and they were given an appointment time. The programme at first focused on the routine child health immunisation programme but it worked so well that the model was extended to HPV vaccinations.
This approach has been extremely well received by families. Comments received included: ‘Thank you so much for today; it was an absolute breeze, no fuss, clear instruction and quick and efficient service.’ ‘’X... was super anxious beforehand but the school nurses were all so kind and welcoming and they put him completely at ease. Fabulous team.’ In the context of the Coronavirus, being able to offer the service in the open air gave parents more confidence than a traditional clinic setting.
It is too soon for data analysis but the school nursing service is confident that immunisation uptake and coverage is much better than it would have been due to restrictions of the Coronavirus. This approach has now gone ‘viral’ with numerous UK-wide children and young people immunisation providers adopting this model of practice as well as other health services such as asthma check-ups for children.

